Welcome back!
Did you have fun with the chalk chromatography experiment?
What colors were in the coating of your chocolate lentils?
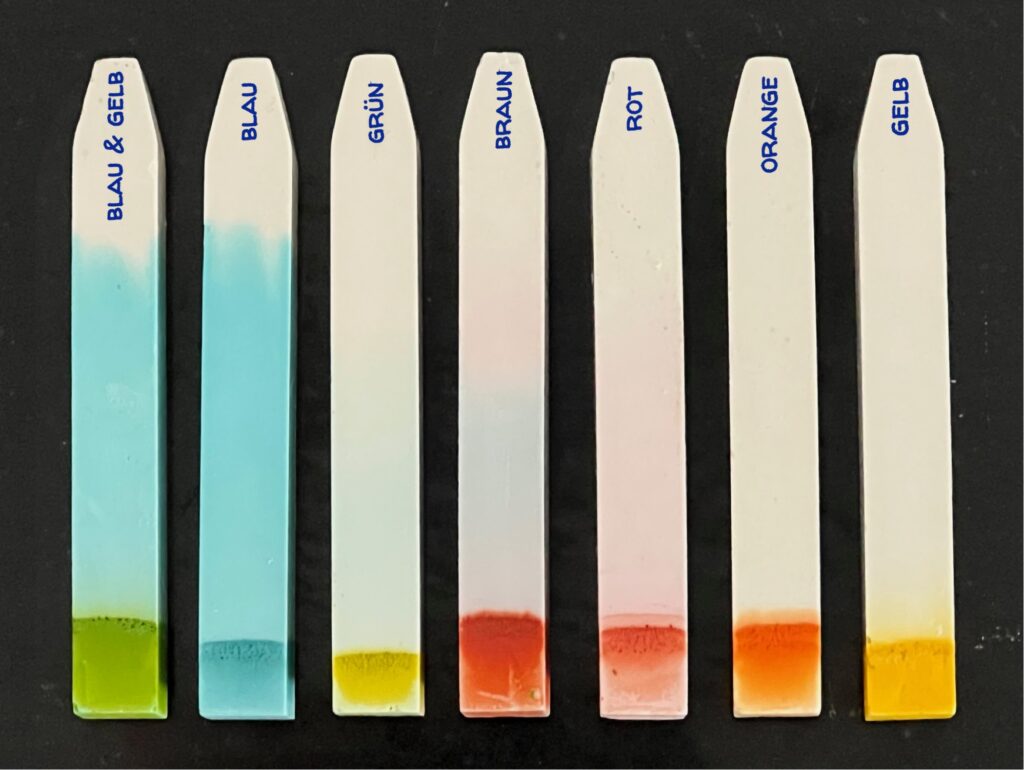
If you didn’t do the experiment, you can still see the result of the color migration (the chromatogram) on the chalk pieces here:
As you can see, you can also separate the colors of chocolate lentils chromatographically.
Even if you mix two colors and they appear as a new shade in the solvent (water), you can separate them again afterwards. You can see this with the mixture of yellow and blue chocolate lentils here:
In this picture, you can see which chromatograms the colors of the chocolate lentils have left on the chalk. (The chalk is labeled with the color of the chocolate lentils.)
Task:
You can surely answer these questions now.
How does the color migration on the chalk work?
The chalk consists of many small particles that have been pressed together firmly, forming the stationary phase. The water can then be drawn up by it, taking the soluble components with it. The water and the soluble components of the colors together form the mobile phase and move through the chalk. This could go on forever if gravity didn’t counteract the whole thing. Therefore, the heavy and large particles get “stuck” first, while the water continues to climb up the chalk and carries lighter particles further with it.
In the chalk chromatography experiment, you can also see that the blue color has migrated the furthest with the eluent water in the stationary phase chalk. This depends on the polarity of the colors, the polarity of the eluent, and the stationary phase. Colors with higher polarity and therefore higher solubility in water (a polar eluent) migrate further: blue has a higher polarity than yellow and therefore migrates further up the chalk. One can also say that blue dissolves well in water and yellow dissolves poorly in water.
Dye Mixtures
The colors we see are the parts of the light spectrum that are reflected by a substance, meaning they are not absorbed. Something appears black to us if it absorbs almost all colors and reflects almost none. The primary dyes are not converted into a new dye when mixed, but superimpose each other in the mixed color, so that we see the mixed color. The color particles have different adhesive forces, which is why they stick at different points on the chalk (or, in paper chromatography, on the filter paper). This is why they can be separated chromatographically.

By the way: Paper chromatography is a “planar” chromatography in 2D format, whereas the piece of chalk represents a simple form of a “column” in 3D format.
Column chromatography and gas chromatography also take place in a column. In the mission to the HPLC, you will learn about a more complex separation column.

Task:
You have now seen the chalk chromatography experiment and can complete our blog assignment.
See you then and good luck!
