How exactly does HPLC work?
Hello!
Were you able to solve the blog task?
Then you are surely ready for a more detailed explanation of HPLC.
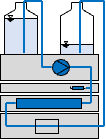
High-performance liquid chromatography (or HPLC) is an analytical method based on a separation process at high pressure in a column.
HPLC is suitable for the separation and identification of substances and can be used both analytically (in the laboratory) and preparatively (for large-scale purification of substances).
If you want to learn more about the areas of application, check out the Excursus: Areas of Application of Chromatography.
In HPLC, a sample is also dissolved in a mobile phase and transported over a stationary phase.
The mobile phase is the so-called “mobile phase” and constantly flows through a solid, finely porous carrier material in the separation column.
The sample is injected into the mobile phase by an injector only at one point in time. The separation effect in HPLC is based on the different polarities of the stationary phase (column) and the mobile phase (eluent). The different molecules in the sample interact with the carrier material of the stationary phase, i.e., they adhere to it and detach again. The stronger the interactions, the more the substances are held back on their way, thus moving more slowly. The sample must be soluble in the mobile phase, which is why there are different mobile phases such as isopropanol for chlorophyll. A suitable mobile phase must be found for each separation task.
The liquid mobile phase must be free of air for HPLC. Air bubbles disrupt the uniform flow of the mobile phase, amplify disturbances in the separation in the column, and interfere with the signals reaching the detector. Therefore, there is a degasser that can remove air bubbles from the system.
Before the column, the sample often passes through a pre-column, which protects the column from contaminants and air bubbles that were not removed by the degasser.
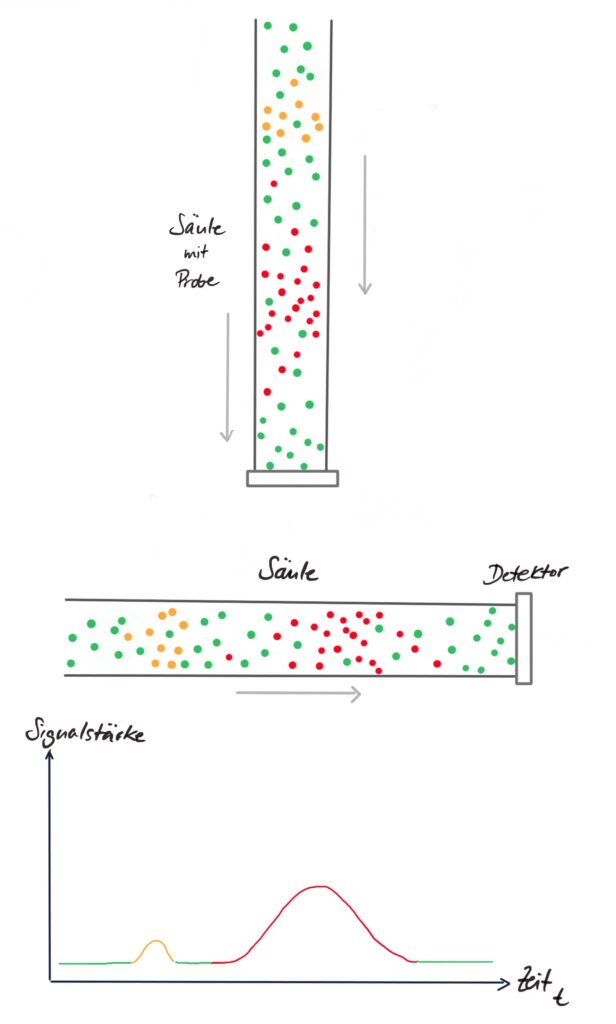
The separation column consists of a tube filled with fine grains, the so-called “stationary phase“. Depending on the task, the grains in the column can be made of very different materials, such as pure sand or plastics. They are chosen so that the different substances from the sample adhere to them to varying degrees but also detach again through the mobile phase. Therefore, the different substances reach the detector at the end of the column at different times.
In the image, you can see how the different components of the sample (colored dots) separate from each other within the column. (So these are not the grains the column is filled with!) When these components reach the detector, the diagram below is generated from them.
There is an experiment by the KinderForscher (Children’s Researchers) at TUHH, together with the Knauer Entdecker Klub Berlin, related to the separation column, where you can build your own separation column to separate large and small balls (“molecules”) from each other. You can find more information about this in this Excursus.
The detector at the end of the separation column makes the separation measurable and visible on a diagram. The detector measures a property of what is currently flowing past it – depending on the type of detector, this could be, for example, color or electrical conductivity, in any case, a property in which the sample and mobile phase differ.
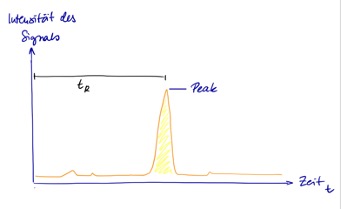
The detector converts the information into electrical signals, which are output on a chromatogram. If only the mobile phase flows past the detector, nothing or only a small value is measured. The more of the substance reaches the detector, the higher the measured value. The signals are recognizable on the chromatogram as peaks. The concentration in the sample can be calculated from the height or the area of the peak. To do this, the chromatogram is compared with diagrams of known substances in known concentrations. Furthermore, the time after which the respective substance arrives at the detector, the so-called retention time, is important, as it is used to identify the substances.
In the picture, you can click on the hotspots to learn more about the parts of an HPLC system.
Have fun!
So, now you know how an HPLC system works.
You can now complete two blog tasks on HPLC and chromatography. Have fun!
Finally, we would like to take you on a tour through the laboratories at ITB (Institute for Technical Biocatalysis) at the Hamburg University of Technology, where HPLC is used. The ITB is introduced at the beginning of the film, HPLC is shown in detail from minute 2:50, and finally, gas chromatography is explained from minute 7:30. Click on the image to start the video!

If you are interested in which professions HPLC is applied in and how it is used in industry, take a look at our Excursuses. There you will also find out in which degree programs you can delve deeper into chromatography and HPLC. We have also collected other links on this page on the topic of chromatography.
Have fun!
